The hapticity of the acenaphthylene ligand in its mononuclear, binuclear, and trinuclear iron carbonyl complexes†
Abstract
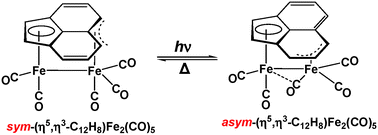
The structures and energetics of the acenaphthylene iron carbonyl complexes (C12H8)Fe(CO)n (n = 4, 3, 2), (C12H8)Fe2(CO)n (n = 8, 7, 6, 5, 4), and (C12H8)Fe3(CO)n (n = 9, 8), including the known (η2-C12H8)Fe(CO)4 and (η5,η3-C12H8)Fe2(CO)5, have been examined using density functional theory. The two experimentally known (η5,η3-C12H8)Fe2(CO)5 structures, related by a thermally reversible photochemical haptotropic rearrangement, lie within ∼1 kcal mol−1 from each other in energy and more than 20 kcal mol−1 below any other (C12H8)Fe2(CO)5 isomers. Decarbonylation of the pentacarbonyl to the tetracarbonyl (C12H8)Fe2(CO)4 is predicted to retain the formal Fe–Fe single bond but to convert one of the CO groups to a four-electron donor bridging η2-μ-CO group. The lowest-energy carbonyl-rich (C12H8)Fe2(CO)n (n = 8, 7, 6) structures lack direct iron–iron bonds and have the ligand hapticities expected from the 18-electron rule. The low CO dissociation energy of ∼5 kcal mol−1 for (C12H8)Fe2(CO)6 suggests that these carbonyl-rich species are not viable. The addition of an Fe(CO)3 moiety to the binuclear (C12H8)Fe2(CO)n (n = 6, 5) complexes to give the trinuclear (C12H8)Fe3(CO)n (n = 9, 8) complexes, respectively, is predicted to be exothermic by ∼50 kcal mol−1. These trinuclear complexes have pentahapto–bis(trihapto) acenaphthylene ligands with an Fe–Fe bond in the octacarbonyl structures. The experimental mononuclear (η2-C12H8)Fe(CO)4 structure with a dihapto acenaphthylene ligand is the lowest energy isomer. The hapticity of the acenaphthylene ligand increases from dihapto to pentahapto or hexahapto upon CO loss to give (C12H8)Fe(CO)n (n = 3, 2).

 Please wait while we load your content...
Please wait while we load your content...