A combined strategy for the synthesis of double functionalized α-zirconium phosphate organic derivatives†
Abstract
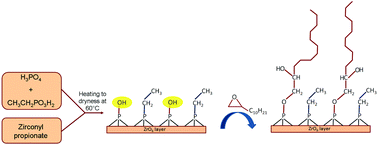
Zirconium phosphate ethylphosphonates, Zr(O3POH)2−x(O3PC2H5)x (hereafter ZrP(PC2)x), with x = 0.6–1.6, were first synthesized from the mixtures of zirconyl propionate, phosphoric and phosphonic acids in THF, and then functionalized by reaction with 1,2-epoxydodecane, C12H24O, at 60 °C. Thermogravimetric analysis together with 31P NMR analysis proved that only the P–OH groups of ZrP(PC2)x quantitatively reacted with the epoxy functionality, leading to the formation of P–O–C bonds. The interlayer distance of the zirconium organophosphate ethylphosphonates, Zr(POC12)2−x(PC2)x, changes continuously from 27.2 Å (for x = 0.6 and 2 − x = 1.4) to 16.6 Å (for x = 1.4 and 2 − x = 0.6), indicating that the ethylphosphonate and organophosphate functionalities are randomly distributed on the layers. The sample with composition Zr(POC12)0.9(PC2)1.1 was used to prepare a polyethylene (PE) based composite containing 5 wt% of filler. Preliminary characterization of the composite was carried out by X-ray analysis, thermogravimetric and DSC measurements. A delay of the thermal decomposition of the polymer up to 40 °C and a shift of the polymer melting temperature toward lower values was observed in the presence of the filler, indicating that a significant polymer–filler interaction occurred when alkyl chains of different lengths are interdispersed on the layer surface.

 Please wait while we load your content...
Please wait while we load your content...