Scope and limitations of two model prebiotic routes to tetrapyrrole macrocycles
Abstract
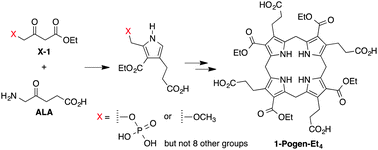
Two routes have been proposed as chemical models for the prebiogenesis of tetrapyrrole macrocycles. In each case, the formation of a pyrrole equipped for self-condensation has proved to be the key step, given that the pyrrole self-condensation readily affords porphyrinogen macrocycles. The scope of the two routes is investigated herein. In the first route, a β-ketoester bearing a 4-phosphonooxy substituent was found to react with δ-aminolevulinic acid (ALA) at lower temperature (35 °C) than that of the 4-methoxy substituent (90 °C) to give the corresponding tetraalkyl-tetraester porphyrin, but eight other 4-substituents (Cl-, HO-, AcO-, AcS-, NCS-, MeS-, chloropyridinium-, and morpholino-) failed at 60 or 90 °C. In the second route, reaction of 1,5-dimethoxypentane-2,4-dione (bearing an acetic acid substituent at the 3-position) with 1-aminoacetone gave the corresponding octaalkylporphyrin in a yield comparable to that of the α-aminoketone ALA. Each pyrrole in the resulting porphyrin bears one methyl group and one acetic acid group, thereby constituting a des-methyl homologue of coproporphyrin. The results delineate the features of structure-directed reactions and highlight the limitations of such reactions where no explicit enzyme-like catalyst is present to guide the outcome.

 Please wait while we load your content...
Please wait while we load your content...