Facile fabrication of thermal-control ionic liquid compound based on undecatungstophosphoindic polyoxometalate with fast ionic conductivity
Abstract
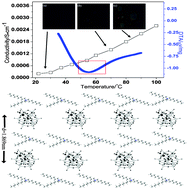
A novel type of POM-type ionic liquid bearing trioctylmethylammonium (TOAMe) and undecatungstophosphoindic polyoxometalate, [TOAMe]4[In(H2O)PW11O39], groups was formed as a reversible-thermal-response gel-format ionic liquid. The compound possessed a layer-type structure and could achieves fast ionic conductivity as high as 2.2 × 10−3 S cm−1 at 90 °C. Interestingly, the conduction of this gel-format compound is highly related to its state, and it exhibits an obvious increase in its conductivity during its reversible phase transformation from a gel state to a liquid–sol state. This compound thus represents a novel type of thermal-control gelation electrolyte. In the range of measured temperatures, the conductivity of [TOAMe]4[In(H2O)PW11O39] increases with higher temperature, with a conductive activation energy for the reversible phase transformation step of 30.19 kJ mol−1.

 Please wait while we load your content...
Please wait while we load your content...