Synthesis of functionalized dihydro-2-oxypyrroles and tetrahydropyridines using 2,6-pyridinedicarboxylic acid as an efficient and mild organocatalyst†
Abstract
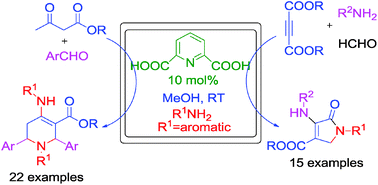
Simple and efficient protocols have been developed for the synthesis of diversely functionalized dihydro-2-oxypyrroles and tetrahydropyridines. One-pot four-component reaction of dialkyl acetylenedicarboxylates, amines and formaldehyde in the presence of 2,6-pyridinedicarboxylic acid in methanol at room temperature provides dihydro-2-oxypyrroles. The combination of β-ketoesters, aromatic aldehydes and amines yielded tetrahydropyridine derivatives under the same reaction conditions. The salient features of these methods are mild reaction conditions, reduced reaction time, moderate to high yields, applicability to a broad range of substrates and no tedious column chromatographic separation.

 Please wait while we load your content...
Please wait while we load your content...