Synthesis of benzothiadiazole-based molecules via direct arylation: an eco-friendly way of obtaining small semi-conducting organic molecules†
Abstract
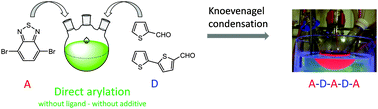
New A–D–A–D–A unsophisticated small molecules based on benzothiadiazole and thiophene were designed and synthesized in a two-step pathway, involving the coupling of benzothiadiazole with thiophene-, or bithiophene-carboxaldehyde, followed by Knoevenagel condensation. The first step was specifically investigated in comparing different coupling methods and their related green metrics. It is established that the synthesis of benzothiadiazole-based small molecules via direct heteroarylation may be an environmentally attractive procedure. It generates a low amount of polluting waste in a cost-effective way in comparison to the classical Suzuki coupling. Moreover, the direct arylation using low loading of the palladium-catalyst without a ligand or an additive is the method of choice for the present case. The physico-chemical properties of the four target molecules were investigated using electronic absorption spectroscopy, differential scanning calorimetry, cyclic voltammetry, polarized optical microscopy and small angle X-ray scattering analysis. DFT studies have been performed to support the electronic characteristics.

 Please wait while we load your content...
Please wait while we load your content...