Structure, formation, thermodynamics and interactions in 9-carboxy-10-methylacridinium-based molecular systems†
Abstract
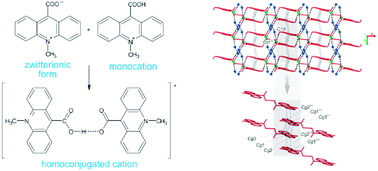
9-Carboxy-10-methylacridinium chloride and trifluoromethanesulfonate, the parent compounds for a wide range of chemiluminogenic salts of practical importance, were synthesized and thoroughly investigated to address problems concerning structural and thermodynamical issues of these cognitively interesting molecular systems. Under various conditions of crystallization, the title salts disclosed three types of crystals: one built from the monomeric form of cations and two containing homoconjugated cations. The title compounds made the first described derivatives of acridine, expressing homoconjugated cationic forms, both in crystalline solid and gaseous phases. The monocrystals were characterized, employing X-ray crystallography and spectroscopic methods such as MALDI-TOF MS, ESI-QTOF MS, NMR and UV-Vis. X-ray crystallography studies revealed the occurrence of the three different molecular architectures, in which not only the counter ions and stoichiometry are different, but also the space group and number of molecules in the unit cell. The energetics and intermolecular interactions occurring within the crystals were explored, applying crystal lattice energy calculations and Hirshfeld surface analysis. In order to elucidate the thermodynamics and origin of the experimentally revealed forms, computations based on the density functional theory were performed, assuming vapour and liquid phases.

 Please wait while we load your content...
Please wait while we load your content...