An efficient water-soluble surfactant-type palladium catalyst for Suzuki cross-coupling reactions in pure water at room temperature†
Abstract
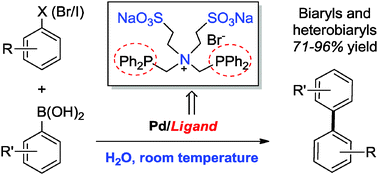
A palladium catalyst based on a bidentate phosphine-type zwitterionic surfactant as a ligand exhibited an excellent catalytic activity in the Suzuki–Miyaura cross coupling reactions. This novel method allowed the reaction of aryl halides with arylboronic acids to occur in pure water at room temperature, forming a variety of biaryls in good to high yields. Heterobiaryls were also efficiently assembled even in the presence of water-insoluble heteroaryl halides as substrates. In addition, such a coupling protocol was successfully used in the iterative diarylation of 2,5-dibromopyridine in one-pot.

 Please wait while we load your content...
Please wait while we load your content...