Fluorescent silver nanoclusters (Ag NCs) in the metal–organic framework MIL-101(Fe) for the catalytic hydrogenation of 4-nitroaniline†
Abstract
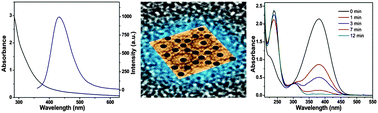
A fluorescent Ag nanocluster deposited iron based metal organic framework, Ag NC@MIL-101(Fe), has been synthesized by a solution impregnation method (MIL stands for Material from Institut Lavoisier). Silver salt solution was impregnated into the porous MIL-101(Fe) and reduced chemically to Ag nanoclusters. The uniform size and high dispersibility of Ag nanoclusters were characterized by UV-vis and photoluminescence spectroscopy, transmission electron microscopy (TEM) and powder X-ray diffraction (XRD). Herein, it is revealed that the stable and monodisperse ultrasmall Ag nanoclusters as small as less than 1 nm in diameter are deposited in the porous MIL-101(Fe). These clusters exhibit highly efficient blue luminescence including a narrow emission profile, a larger Stokes shift and high photostability upon excitation at 330 nm. The as-synthesized Ag NC@MIL-101(Fe) exhibits enhanced catalytic activity towards the heterogeneous reduction of 4-nitroaniline to 4-phenylenediamine under green and ambient conditions. Furthermore, the catalyst can be reused for five consecutive runs with significant recycling stability over 90%. Our results suggest that these fluorescent clusters can be used in green heterogeneous synthesis.

 Please wait while we load your content...
Please wait while we load your content...