Exploring the reaction pathways of Pd(ii)-catalyzed cyclohexene oxidation with molecular oxygen: vinylic and allylic oxidation, disproportionation and oxidative dehydrogenation
Abstract
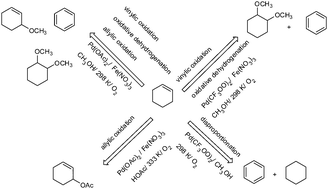
Palladium(II) salts are able catalysts to promote different oxidative transformations of cyclohexene in the presence of molecular oxygen: vinylic and allylic oxidation and disproportionation. In this paper, we assessed the main aspects that govern these reactions, by using palladium salts (i.e. PdCl2, Pd(OAc)2, Pd(acac)2 and Pd(CF3COO)2) in protic solvents (i.e. CH3COOH and CH3OH). When carried out in CH3COOH solutions at 333 K, the Pd(II)-catalyzed oxidation reactions preferentially convert cyclohexene to 2-cyclohexenil-1-acetate. Benzene was the secondary product. It was found that the efficiency of the palladium reoxidant followed the trend: Fe(NO3)3 > LiNO3 > CuCl2 > FeCl3. Pd(OAc)2 was the most active catalyst. Replacing Cu(OAc)2 by Fe(NO3)3 notably enhances the conversion of cyclohexene and the selectivity of 2-cyclohexenyl-1-acetate, reducing significantly the reaction time from 22 to 3 h. Conversely, when performed in CH3OH solutions, the reaction had its selectivity drastically changed; benzene and cyclohexane were the most selectively formed products (i.e. through disproportionation reaction). In this case, the reaction occurred at room temperature and in the absence of a reoxidant. Hence, Pd(CF3OO)2 is the most active and selective catalyst. The addition of the Fe(NO3)3 reoxidant and increasing the reaction temperature to 328 K resulted in the formation of 1,2-dimethoxycyclohexane and benzene as major products (i.e. allylic oxidation and oxidative dehydrogenation products, respectively). Catalytic cycles for these transformations were proposed based on experimental data and palladium chemistry.


 Please wait while we load your content...
Please wait while we load your content...