Benzophenone-imbedded benzoyltriptycene with high triplet energy for application as a universal host material in phosphorescent organic light-emitting diodes (PhOLEDs)†
Abstract
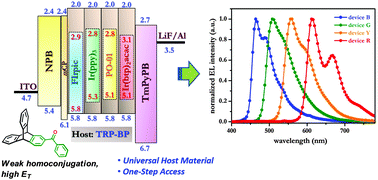
Benzoyltriptycene (TRP-BP) – accessed readily by Fridel–Crafts benzoylation reaction – represents a molecular system with an imbedded benzophenone chromophore. The concave features inherent to a triptycene scaffold impart amorphous properties, which is an important consideration in OLEDs. Due to very weak homoconjugation in TRP-BP, the high triplet energy of the parent benzophenone chromophore (ca. 2.99 eV) is conserved. This in conjunction with a high band gap (3.80 eV) permits application of TRP-BP as a universal host material for blue, green, yellow and red dopants. An external quantum efficiency of 19.8% and the brightness of 11 800 cd m−2 were elicited from the simple devices fabricated with yellow and green dopants, namely, PO-01 and Ir(ppy)3 respectively. Ready synthetic access and impressive device performance results make TRP-BP an important addition to the scantily explored host materials based on benzophenone, which has traditionally served as a prototype carbonyl compound in the development of organic photochemistry in general to delineate the triplet-excited state properties.

 Please wait while we load your content...
Please wait while we load your content...