2,4 and 2,5-bis(benzooxazol-2′-yl)hydroquinone (DHBO) and their borate complexes: synthesis and optical properties†
Abstract
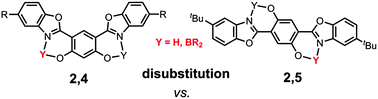
This article describes the multistep synthesis and the solution/solid-state optical properties of a series of highly fluorescent dyes based on either a 2,4 or a 2,5-bis(benzooxazol-2′-yl)hydroquinone (DHBO) scaffold. Their fluorescence emission stems from an intrinsic excited-state intramolecular proton transfer (ESIPT) photophysical process, which is highly dependent on the environment of the probe (solvent/matrix). Besides their ESIPT emission, these dyes are also excellent chelates towards boron(III) fragments such as BF2 or B(Ar)2 leading to singlet emitters with a quantum yield in solution reaching up to 51%. The solid-state emission of all these fluorophores was investigated by dispersing them as pellets in a KBr matrix revealing an intense emission in condensed matter.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...