Switchable two-photon imaging of RGD-functionalized polynorbornenes with enhanced cellular uptake in living cells†
Abstract
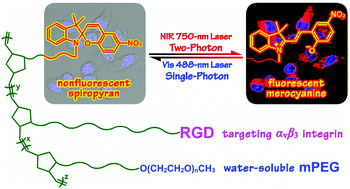
A series of random copolymers PNB–RGDx-co-SPy-co-mPEGz were fabricated directly from three orthogonal modules, photochromic spiropyran, RGD peptides and hydrophilic polyethylene glycol (PEG) via a modular ROMP (ring-opening metathesis polymerization) strategy. Attributed to the incorporation of integrin-recognition RGD peptides, these polymeric spiropyran probes exhibited excellent biocompatibility and target-binding specificity towards integrin positive human hepatoma Bel-7402 tumor cells. Compared to the partially cationic ammonium analogue, RGD-functionalized spiropyran copolymers showed greatly enhanced efficiency in cellular uptake, whose fluorescence was also switchable between the fluorescent merocyanine (MC) ON state and the non-fluorescent spiropyran (SP) OFF state in living cells upon alternating near-infrared (NIR) 780 nm two-photon and visible 488 nm single-photon laser excitations. Flow cytometric analysis and zeta potential measurements revealed that the RGD-functionalized copolymers possess similar surface potentials to that of the ammonium analogue; a positive charge benefits but does not dominate the intercellular translocation of these polymeric fluorescence probes. The results provide bioactive peptide-functionalized copolymers with significant promise for targetable two-photon imaging applications in the fields of cancer diagnosis and therapy.

 Please wait while we load your content...
Please wait while we load your content...