Facile synthesis of new N-sulfonamidyl-4-thiazolidinone derivatives and their biological evaluation†
Abstract
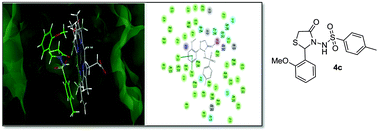
The one-pot three-component syntheses of new N-sulfonamide-thiazolidin-4-one derivatives were carried out in excellent yield using [HDBU][HSO4] as an ionic liquid under solvent-free conditions. The newly synthesized compounds were screened against fungal strains and a number of compounds were seen to display excellent antifungal activity. In addition, the synthesized compounds were screened for their scavenging activity of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical and showed very good antioxidant activity. Finally, theoretical predictions derived from molecular docking studies against the potential target sterol 14α-demethylase (CYP51) helped establish a link between the observed biological activity and the binding affinity, thereby providing insights into the specific bonding and non-bonding interactions governing the activity.

 Please wait while we load your content...
Please wait while we load your content...