Unexpected formation of 4,4-dimethyl-1,2-disubstituted-dicarbonyl cyclopentanes from ketone enolate anions and 1,3-diiodo-2,2-dimethylpropane†
Abstract
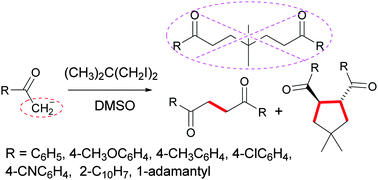
Cyclic 1,4-dicarbonyl compounds can be easily obtained by mixing a solution of aryl methyl or alkyl methyl ketone enolate anions with 1,3-diiodo-2,2-dimethylpropane in DMSO. This represents the first example of dimerization of ketone enolate anions using a simple diiodoalkane as a reagent followed by subsequent double alkylation with bis-iodide yielding a cyclopentane adduct. This methodology allows the use of a simple potassium tert-butoxide as a base at room temperature for the formation of three C–C bonds resulting in a relatively complex five-membered ring diketone structure under transition-metal-free conditions.

 Please wait while we load your content...
Please wait while we load your content...