Energetic contribution to both acidity and conformational stability in peptide models†
Abstract
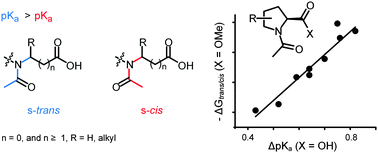
The acidity of N-acyl amino acids is dependent upon the rotameric state of the amide bond. In this work we systematically investigated the acidity difference of the rotamers (ΔpKa) in the frames of various acetylated amino acids. Our results indicated a mutual interaction of two carbonyl groups of an attractive type. We observed conservative ΔpKas for acyclic amino acids (2.2–3.0 kJ mol−1), whereas in the case of alicyclic amino acids, the experimental values revealed a strong dependency on the structural context (1.5–4.4 kJ mol−1). In homologous amino acids (α-, β-, γ-, etc.), the strength of the attraction decays in an exponential fashion. Furthermore, the interaction can accumulate through a chain of amide bonds in a cascade fashion, as demonstrated by an Ac-Pro-Pro dipeptide. As a result, we demonstrate that ΔpKa is an experimental parameter to estimate increments in the carbonyl–carbonyl alignment, as determined by the amino acid or peptidyl context. This parameter is also important in understanding the roles of amino acids in both protein folding and translation in biological systems as well as their evolutionary appearance in the genetic code.

 Please wait while we load your content...
Please wait while we load your content...