Green one step morphosynthesis of silver nanoparticles and their antibacterial and anticancerous activities†
Abstract
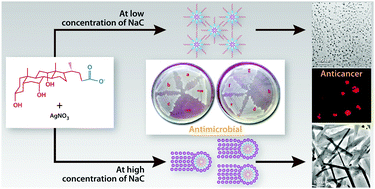
Bile salts carry out a vital bioactive responsibility among the various physiologically important molecules. The use of bile acids and their conjugates in nanoscience is a novel idea, which opens up fascinating prospects and gives rise to various versatile properties. Sodium cholate, a biosurfactant which is environmentally safe, has been used to reduce and stabilize silver nitrate solution. The silver nanostructures thus produced are crystalline and anisotropic in nature and show different types of particle shapes and sizes depending on the reaction conditions including star and wire shapes which have not been reported earlier, by simply varying the reaction conditions. The silver precursor concentration and the ratio of sodium cholate to silver precursor were the key factors which controlled the morphosynthesis. The formation of the silver NPs was monitored using UV-vis spectrophotometry. The transmission electron microscopy (TEM) technique was used to study the morphology of the synthesized NPs. Fourier transform infrared (FT-IR) spectroscopy was used to identify the interaction of sodium cholate with the synthesized NP. The possible mechanistic role of sodium cholate in the reduction and stabilization of silver nanoparticles (Ag NPs) has also been discussed. The obtained Ag NPs exhibit antibacterial and anticancerous activities. The present study also explores the cytotoxic role of Ag NPs in KG-1A (human acute myeloid leukemia) and K562 (human chronic myeloid leukemia) cell lines.

 Please wait while we load your content...
Please wait while we load your content...