Synthesis and spectroscopic properties of β,β′-dibenzo-3,5,8-triaryl-BODIPYs†
Abstract
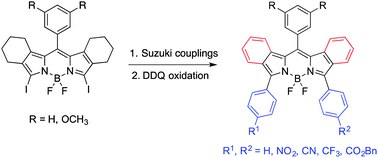
A series of β,β′-bicyclo-3,5-diaryl-BODIPYs were synthesized from the corresponding β,β′-bicyclo-3,5-diiodo-BODIPYs (1a,b) via Pd(0)-mediated Suzuki cross-coupling reactions in 82–92% yields. Subsequent aromatization with DDQ afforded the corresponding β,β′-dibenzo-aryl-BODIPYs, which showed red-shifted absorptions and emissions in the near-IR range. The dibenzo-appended BODIPYs showed characteristic 1H-, 13C-, 11B- and 19F-NMR shifts, and nearly planar conformations by X-ray crystallography.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...