Oxidative cleavage of 1-aryl-isochroman derivatives using the Trametes villosa laccase/1-hydroxybenzotriazole system†
Abstract
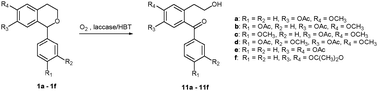
The oxidative cleavage of the dihydropyran ring of 1-aryl-isochroman derivatives was carried out for the first time under green chemistry conditions in the presence of the Trametes villosa laccase/1-hydroxybenzotriazole system in buffered water/1,4-dioxane and buffered water/dimethyl carbonate as reaction media. The corresponding oxidation products [2-(2-hydroxyethyl)benzophenone derivatives] were obtained in different yields depending on the substituents on phenyl and isochroman rings. These compounds are useful intermediates for the synthesis of anticancer agents and neuroprotective drugs.


 Please wait while we load your content...
Please wait while we load your content...