α-Hydroxyacids accelerate the Diels–Alder reaction of dibutyl vinylboronate with cyclopentadiene: experimental results and mechanistic insights†
Abstract
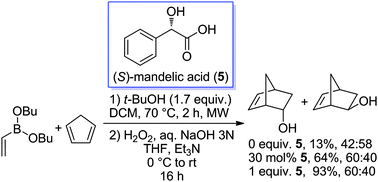
We have found that α-hydroxyacids accelerate the Diels–Alder reaction of dibutyl vinylboronate with cyclopentadiene. When stoichiometric quantities are used, excellent yields are obtained, while catalytic activities are moderate. DFT calculations suggested that the activation of the dienophile occurs by ligand exchange with both functionalities of the α-hydroxyacid.

 Please wait while we load your content...
Please wait while we load your content...