Fabrication and study of optical and electrochemical properties of CdS nanoparticles and the GO–CdS nanocomposite
Abstract
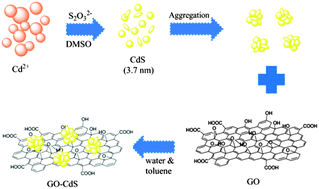
CdS nanoparticles (NPs) have been successfully synthesized using the cadmium(II)–salophen complex and anhydrous sodium thiosulfate (Na2S2O3) as a sulfur source in dimethyl sulfoxide (DMSO) via a thermal deposition approach. The average size of the CdS NPs is 3.7 nm. The graphene oxide (GO)–CdS nanocomposite was successfully prepared via a facile process. The as-prepared nanocomposite possesses excellent optical and electrochemical properties. The structure, morphology, optical and electrochemical properties of the CdS NPs and the GO–CdS nanocomposite were studied by X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), energy-dispersive X-ray spectroscopy (EDS), UV-Vis diffuse reflectance spectroscopy (DRS), photoluminescence (Pl) spectroscopy, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). UV-Vis spectra show that compared to CdS NPs, the absorption edge of the GO–CdS nanocomposite is red shifted slightly and the absorption intensity is decreased. Photoluminescence spectra of CdS NPs consist of an emission peak which is centered at around 510 nm, when excited at 325 nm. It is noteworthy that the blue luminescence intensity of the GO–CdS nanocomposite is lower than that of pure CdS NPs. The electrochemical observation of samples suggests that the CdS nanoparticles integrate with GO sheets and thereby facilitate the electron transfer. Furthermore, the possible growth mechanism of CdS nanoparticles is presented.

 Please wait while we load your content...
Please wait while we load your content...