SBA-15 functionalised with high loading of amino or carboxylate groups as selective adsorbent for enhanced removal of toxic dyes from aqueous solution†
Abstract
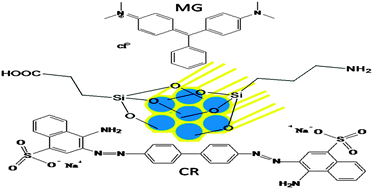
Amino or carboxylic acid group-containing SBA-15 materials were prepared after activation of surface silanol groups using concentrated HCl. As a result, grafting of amino or carboxylic acid groups on SBA-15 occurred more effectively, due to the presence of active surface silanol groups (3.09 mmol g−1 of SBA-15-NH2, 2.16 mmol g−1 of SBA-15-COOH). These materials can selectively remove toxic dyes (SBA-15-NH2 removes congo red, CR and SBA-15-COOH removes malachite green, MG) from aqueous solution. Various instrumental techniques like DTA, elemental analysis, FTIR, nitrogen adsorption–desorption isotherms, HRXRD, FESEM and HRTEM were employed to examine the pre- and post-adsorption surface morphology. The adsorption behaviour of the nano adsorbents toward toxic dyes was evaluated by examining the effect of pH, solution temperature, time, adsorbent dose, dye concentration, and agitation speed. The obtained kinetic and thermodynamic data fitted best with a pseudo-second-order model and the Langmuir model, respectively. Both the SBA-15-NH2 and SBA-15-COOH adsorbents have large and enhanced adsorption capacities (SBA-15-NH2/CR, qm: 230.95 mg g−1; SBA-15-COOH/MG, qm: 390.63 mg g−1) for these toxic dyes. These adsorbents require less maintenance, resulting in low operating cost. Additionally, these materials have efficient regeneration capabilities.

 Please wait while we load your content...
Please wait while we load your content...