Preparation and self-assembly of amphiphilic polylysine dendrons†
Abstract
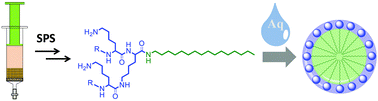
Herein, we present the synthesis of new amphiphilic polylysine dendrons with variable alkyl chain lengths (C1–C18) at the C-terminal. The dendrons were synthesized in moderate to quantitative yields by divergent solid-phase synthesis (SPS) employing an aldehyde linker. The self-assembling properties of the dendrons in aqueous solutions were studied by small angle neutron scattering (SANS) and dynamic light scattering (DLS). The self-assembling properties were influenced by the length of the alkyl chain and the generation number (Gn). Increasing the temperature and concentration did not have significant impact on the hydrodynamic diameter, but the self-assembling properties were influenced by the pH value. This demonstrated the need for positively charged amines in the head groups for the successful formation of controlled self-assemblies. Dendrons having alkyl chains below C8 did not self-assemble. Well-defined micellar structures observed with SANS were formed with alkyl chain lengths above C12. Large structures detected with DLS for dendrons with alkyl chain lengths above C12 are ascribed to intermicellar aggregates stabilized by hydrophobic and electrostatic forces in accordance with the observed pH effect. Finally, the cytotoxicity of the dendrons was evaluated in mouse fibroblast (NIH/3T3) and human embryonic kidney (HEK 293T) cells at 5, 10 and 20 μM concentrations. The dendrons showed low cytotoxicity, displaying cell viability well above 80%.

 Please wait while we load your content...
Please wait while we load your content...