Biosourced 1,2,3-triazolium ionic liquids derived from isosorbide†
Abstract
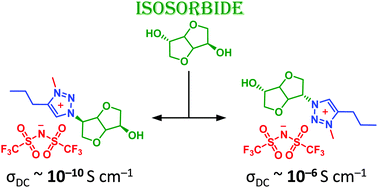
Two series of 1,2,3-triazolium chiral ionic liquid (CIL) stereoisomers are synthesized from isosorbide by combining the robust attributes of the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC), alkylation of the resulting 1,2,3-triazoles by methyl iodide and further anion metathesis with different fluorinated salts. These novel biosourced CILs are characterized in details by 1H and 13C NMR spectroscopy, high-resolution mass spectrometry (ESI-HRMS), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and broadband dielectric spectroscopy (BDS). The effect of stereochemistry on their physical, thermal and ion conducting properties is discussed. Markedly, it is demonstrated for the first time that stereochemistry can drastically influence ionic conductivity by several orders of magnitude.

 Please wait while we load your content...
Please wait while we load your content...