TD-DFT and structural investigation of natural photosensitive phenanthroperylene quinone derivatives†
Abstract
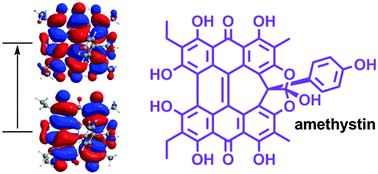
Natural product derivatives of phenanthro[1,10,9,8-opqra]perylene-7,14-dione (PPD) are important potential agents in photodynamic therapy. Density functional theory (DFT) optimized geometries and time-dependent DFT (TD-DFT) vertical transitions of fringelite D (a pigment from an extinct species of sea lily), hypericin (an active component of Saint John's wort), stentorin C (Stentor coeruleus), blepharismin C (Blepharisma japonicum) and amethystin (Stentor amethystinus) are in good agreement with experimental structural, IR and UV-Vis data. The structure of the newly isolated amethystin has not been fully determined, but comparisons between the experimental and DFT IR frequencies suggest that this compound most likely adopts an orthoester structure. The π → π* excitations in the molecules are traceable to the PPD core, and are bathochromically shifted due to the peri –OH substituents with a smaller effect from the molecular twist induced by bay –Me and/or –OH groups. Hydrogen bonding between the peri –OH groups and the carbonyls is important to the ability of these species to photochemically generate singlet oxygen and superoxide ions.

 Please wait while we load your content...
Please wait while we load your content...