Using non-covalent interactions to direct regioselective 2+2 photocycloaddition within a macrocyclic cavitand†
Abstract
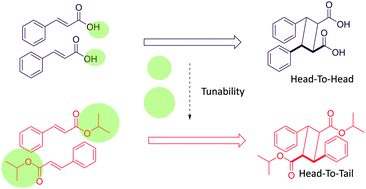
The relative orientation of guests within ternary inclusion complexes is governed by the host–guest and guest–guest supramolecular interactions. Selectivity in 2+2 photocycloaddition between two alkenes included within a macrocyclic cavitand (γ-cyclodextrin) can be controlled using non-covalent interactions. In this manuscript, we report cavitand-mediated control of regioselectivity between alkyl cinnamates using non-covalent interactions. Using this method, we have shown that regioselectivity can be switched completely from a head-to-head dimer to a head-to-tail dimer. The reactions were also stereoselective in most cases. Stoichiometry experiments were performed to explore relative stabilities of the complexes, which indicate that the ternary complex is more stable than others. Selectivity in the photocycloaddition reaction was also applied retrospectively to deduce intermolecular orientations. Time-dependent conversion study we performed indicates that the observed reactivity of alkenes is representative of the intermolecular orientations in the bulk of the complex medium. Experimental observations and computational studies were used to qualitatively understand the complex structures, and relative magnitudes of the weak interactions. The reactions of complexes were studied in slurry form, and the extent of reaction control suggests a solid-state-like behavior.

 Please wait while we load your content...
Please wait while we load your content...