Colorimetric β-lactamase inhibitor assay with double catalyzed signal amplification†
Abstract
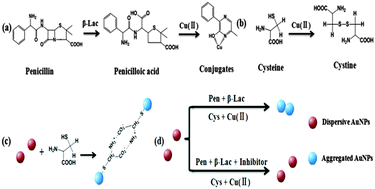
A colorimetric method is developed for the determination of the activity of β-lactamase (β-Lac) and the screening of its inhibitors based on cysteine-induced gold nanoparticle (AuNP) aggregation. The widely used penicillin is served as a β-Lac substrate, and the strong reduction capability of its corresponding product, which can react with Cu(II) to form Cu(I) conjugates, is utilized in the method. The double catalyzed signal amplification is accomplished via the addition of both β-Lac and Cu(II) which can catalyze the oxidization of cysteine into cystine. The activity of β-Lac is determined at levels as low as 2.36 U mL−1. The inhibitory effects of clavulanic acid and sulbactam are tested and give 0.92 and 0.06 μM, respectively. The assay is simple, rapid, easy-operated, and selective, and the proposed method has a great potential not only for the detection of β-Lac activity but also for the screening of the inhibitors.

 Please wait while we load your content...
Please wait while we load your content...