Immobilized Pd(0) nanoparticles on phosphine-functionalized graphene as a highly active catalyst for Heck, Suzuki and N-arylation reactions†
Abstract
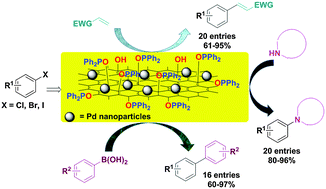
In this study, a phosphine group was chemically grafted to the surface of graphene in order to prepare a reusable ligand with high surface area, incorporating a phosphine moiety. The treatment of graphene oxide (GO) with hydroxide followed by an aqueous work-up yields an OH-functionalized graphene material (GOH) via ring-opening of the epoxide groups. Reaction of GOH with chlorodiphenylphosphine (ClPPh2) gives a new material, GOPPh2 (PFG), which can be used for stabilization of metal nanoparticles or complexation of transition metals in order to prepare a reusable metal catalyst. Stabilization of palladium nanoparticles on the surface of GOPPh2 resulted in the production of an efficient heterogeneous Pd catalyst (PFG–Pd) for application in C–C and C–N bond formation reactions. The PFG–Pd catalyst was characterized using some different microscopic and spectroscopic techniques such as FT-IR, XRD, TEM, SEM, EDX, and ICP analysis. The applicability of the PFG–Pd catalyst was evaluated in Heck, Suzuki and N-arylation reactions. The catalyst system showed high catalyst activity in these processes and the target products were obtained in high isolated yields. The PFG–Pd catalyst was reusable in these reactions for at least 5 times with no significant decrease in its catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...