Boron avoids cycloalkane-like structures in the LinBnH2n series†
Abstract
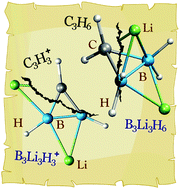
The stability of the LinBnH2n (n = 3–6) series was analyzed using quantum chemical calculations, and it was found that cyclic isomers are not energetically favored. This is different to what happens in their organic counterparts (CnH2n), where cyclopentane (C5H10) and cyclohexane (C6H12) are the low-lying isomers. Apparently, aromaticity is a key-stabilizing factor that needs to be considered for designing stable lithium-boron hydride analogues of cyclic organic compounds. This is verified in the Li3B3H3+ system, which has been designed as the smallest aromatic carbocation (C3H3+) analogue. The global minimum structure of Li3B3H3+ contains a triangular B3H32− moiety, which has structural and chemical bonding features similar to its organic counterpart. Besides, this new cluster is classified as aromatic according to both the 4n + 2 Hückel rule and the analysis of the induced magnetic field. This theoretical evidence leads us to propose this cluster as a viable target for experimental detection in the gas phase.


 Please wait while we load your content...
Please wait while we load your content...