Mimicking vanadium haloperoxidases: vanadium(iii)–carboxylic acid complexes and their application in H2O2 detection†
Abstract
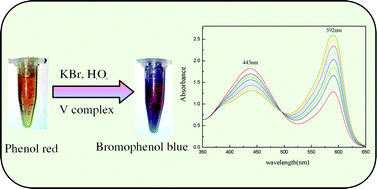
Vanadium(III) complexes [V(2,6-pdc)2(H2O)2]·2H2O (1) and V(2,6-pdc)(htba)(H2O)2 (2), (2,6-pdc = 2,6-pyridinedicarboxylic acid, htba = 2-acetoxy-4-trifluoromethylbenzoic acid) have been synthesized by the reaction of V2(SO4)3 with 2,6-pdc (for 1) or 2,6-pdc and htba (for 2) under hydrothermal conditions at 120 °C for 36 hours. Because the vanadium(III) was easily oxidized into higher oxidation states, we included the reducing agent vitamin C to protect the vanadium(III) center. The complexes were characterized by elemental analysis, IR and UV-vis spectroscopy, and single-crystal X-ray diffraction. Structural analysis revealed that the central metal V atoms in the complexes 1 and 2 were seven-coordinate, forming pentagonal bipyramid geometries. The complexes catalyzed the bromination of the organic substrate phenol red in the presence of H2O2, bromide and buffer. Compared with vanadium complexes having other oxidation states, the vanadium(III) complexes had better catalytic activity (the maximum reaction rate constant was 2.424 × 102k(mol L−1)−2 s−1). The mimicking vanadium haloperoxidases also overcame some serious disadvantages of natural enzymes. Therefore, the reaction system described herein can be considered as an effective model for hydrogen peroxide determination.

 Please wait while we load your content...
Please wait while we load your content...