Enhancement of photochemical heterogeneous water oxidation by a manganese based soft oxometalate immobilized on a graphene oxide matrix
Abstract
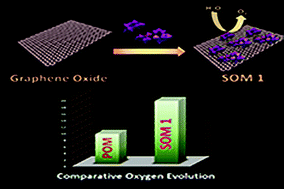
Development of efficient and oxidatively stable molecular catalysts having abundant transition metals at the active site is an immediate challenge to synthetic chemists in order to photochemically split water into clean fuels oxygen and hydrogen to serve the ever-increasing energy demand. Herein we report a soft-oxometalate (SOM)-based heterogeneous photocatalytic system which effectively performs water oxidation giving oxygen. In the present work we placed a double sandwich type manganese-based polyoxometalate (POM), Na17[Mn6P3W24O94(H2O)2]·43H2O, on an electroactive graphene oxide matrix and synthesized a new SOM [Na17[Mn6P3W24O94(H2O)2]·43H2O@graphene oxide] 1 and performed water oxidation with it. The efficiency of photocatalytic water oxidation by SOM 1 is almost double than in the case of Na17[Mn6P3W24O94(H2O)2]·43H2O alone. The rationale behind this lies in the electron accepting nature of the graphene sheets which effectively relay the electrons generated in the water oxidation reaction, thus facilitating the forward reaction and increasing the oxygen yield. Variation of catalyst loading, pH-dependent and time-dependent experiments are performed to study their effects on photocatalytic water-splitting. The reaction kinetics is sigmoidal in nature, suggesting the heterogeneous nature of catalysis. The composite catalyst system is observed to be stable towards the reaction conditions.
- This article is part of the themed collection: Emergent Polyoxometalates and Soft-oxometalates

 Please wait while we load your content...
Please wait while we load your content...