Synthesis and pharmacological evaluation of conformationally restricted κ-opioid receptor agonists†‡
Abstract
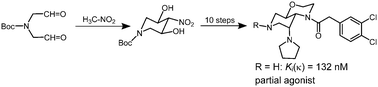
In order to obtain novel polar κ agonists the κ-pharmacophoric ethylenediamine structural element was embedded in a rigid bicyclic scaffold. The pyridooxazine system was selected since it contains polar O- and N-atoms in the 1- and 7-positions, respectively. An axially oriented pyrrolidine ring was attached at the 5-position and the dichlorophenylacetyl moiety was introduced at N-4. The key steps of the 11-step synthesis are a double Henry reaction of iminodiacetaldehyde 7 with nitromethane and the introduction of the azido moiety of 13 by Mitsunobu reaction of the alcohol 11 with Zn(N3)2·(pyridine)2. The X-ray crystal structure analysis of 17b shows a dihedral angle N(acyl)-C-C-N(pyrrolidine) of −60.8(2)°, which is close to the postulated optimal angle. Moderate κ affinity was found for the secondary amine 17a (Ki = 132 nM) and the methyl derivative 17b (Ki = 266 nM). In the [35S]GTPγS assay the secondary amine 17a showed 28% agonistic activity compared to U-69,593. Although 17a and 17b contain all crucial κ-pharmacophoric elements, their κ affinity is rather low, which might be attributed to the unfavorable cis-orientation of the pyrrolidine ring and the dichlorophenylacetamido moiety and/or the additional O- and N-atoms in the 1- and 7-positions.

 Please wait while we load your content...
Please wait while we load your content...