Design, synthesis and biological evaluation of caffeoyl benzanilides as dual inhibitors of HIV integrase and CCR5†‡
Abstract
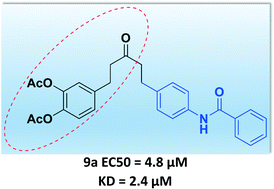
Novel series of caffeoyl benzanilide compounds as dual inhibitors of HIV-1 CCR5/IN were designed and synthesized. The biological results indicated that the acetylated compounds with double bonds were reduced, especially compound 9a, which showed potential activity against HIV-1 CCR5 tropic viruses with an EC50 value of 4.85 μM, as well as binding affinity with IN (KD 2.4 μM). Molecular modeling studies also suggested the possible binding mode of 9a with CCR5 and IN. These results indicated that 9a has the possibility of being a dual inhibitor of HIV-1.

 Please wait while we load your content...
Please wait while we load your content...