Abstract
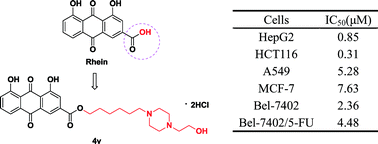
A series of rhein derivatives (4a–x) were synthesized and evaluated for their aqueous solubility and in vitro anti-proliferative activities against six different tumor cell lines. The aqueous solubility of the compounds was in the range of 10.04 to 15.08 mg mL−1, which is 220 to 330-fold higher than that of rhein (0.0456 mg mL−1). All derivatives displayed more potent anti-tumor activity than rhein, and most of them were even stronger than 5-FU, particularly, 4s, 4t and 4v (IC50: 3.01–5.28 μM on A549 cells, 5.92–7.63 μM on MCF-7, 0.33–0.85 μM on HepG2 cells, 0.31–0.83 μM on HCT116, 2.36–6.49 μM on Bel-7402 and 4.48–7.31 μM on Bel-7402/5-FU cells). Compound 4v was further found to induce HCT116 cell apoptosis, and G2/M-phase cell cycle arrest via down-regulation of CDK1 and cyclin B in HCT116 cells. Our findings suggest that 4v could be a promising lead for future studies.

 Please wait while we load your content...
Please wait while we load your content...