Classification of matching molecular series on the basis of SAR phenotypes and structural relationships†
Abstract
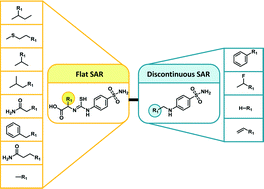
In medicinal chemistry, matched molecular pairs (MMPs) have experienced increasing interest. An MMP is defined as a pair of compounds that only differ by a structural change at a single site. MMPs are often used to associate specific structural modifications with changes in molecular properties. As an extension of the MMP concept, matching molecular series (MMSs) have been introduced. An MMS is defined as a set of compounds with pairwise MMP relationships and thus represents a series of analogs with modifications at a single site. We have aimed to systematically identify publicly available MMSs, characterize the SAR information encoded by MMSs and explore structural relationships between them. Therefore, we have searched for MMSs in more than 700 compound activity classes, systematically explored structural relationships between MMSs, and characterized the structure–activity relationship (SAR) information they contain. Combining SAR and structural relationship information has made it possible to identify structurally related MMSs with similar or distinct SAR characteristics, which frequently shared compounds. Such MMSs combine series of analogs with different substitution sites and reveal how structural modifications influence SARs. They can also be used to explore analog pathways that change SAR characteristics and provide additional SAR information. As a part of our study, a comprehensive database of pairs of MMSs with different SAR characteristics is made freely available for medicinal chemistry applications.

 Please wait while we load your content...
Please wait while we load your content...