Abstract
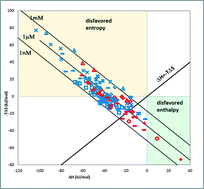
Detailed thermodynamic analysis of fragment binding revealed that unlike drug-like compounds, fragments bind with significant enthalpic preference. This observation is in line with the size dependency of binding enthalpy contributions and is also supported by a large body of experimental data from direct binding thermodynamic measurements. The enthalpy-driven binding of fragments represents a thermodynamic rationale for fragment-based drug discovery programs and suggests guidelines for fragment-based optimization programs.

 Please wait while we load your content...
Please wait while we load your content...