Abstract
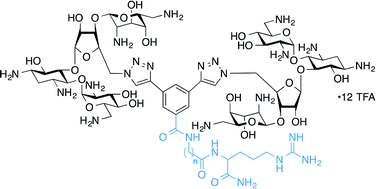
The nucleotides comprising the ribosomal decoding center are highly conserved, as they are important for maintaining translational fidelity. The bacterial A-site has a small base variation as compared with the human analogue, allowing aminoglycoside (AG) antibiotics to selectively bind within this region of the ribosome and negatively affect microbial protein synthesis. Here, by using a fluorescence displacement screening assay, we demonstrate that neomycin B (NEO) dimers connected by L-arginine-containing linkers of varying length and composition bind with higher affinity to model A-site RNAs compared to NEO, with IC50 values ranging from ~40–70 nM, and that a certain range of linker lengths demonstrates a clear preference for the bacterial A-site RNA over the human analogue. Furthermore, AG-modifying enzymes (AMEs), such as AG O-phosphotransferases, which are responsible for conferring antibiotic resistance in many types of infectious bacteria, demonstrate markedly reduced activity against several of the L-arginine-linked NEO dimers in vitro. The antimicrobial activity of these dimers against several bacterial strains is weaker than that of the parent NEO.

 Please wait while we load your content...
Please wait while we load your content...