Effects of polar κ receptor agonists designed for the periphery on ATP-induced Ca2+ release from keratinocytes†
Abstract
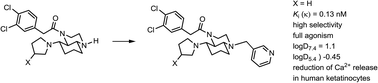
In order to obtain polar κ agonists, which cannot pass the blood–brain barrier, secondary amines 3a and 3b were reductively alkylated with pyridine-3-carbaldehyde to give pyridylmethyl substituted perhydroquinoxalines 5a and 5b, respectively. Both 5a and 5b show very high κ-opioid receptor affinity with Ki values of 0.13 nM and 3.8 nM, respectively. Moreover, they are very selective for the κ receptor. In the [35S]GTPγS assay, both quinoxalines reached full agonistic activity. With an EC50 value of 34 nM, 5a is only slightly less potent than the prototypical κ agonist U-69,593. For the determination of the log D values, a shake-flask method was developed, making use of quantification by mass spectrometry which requires only very small amounts of the compound (<0.8 mg). According to this method, the log D7.4 and log D5.4 values of 5a were 1.1 and −0.45, indicating very high polarity. The log D5.4 value was recorded due the acidic milieu of the skin. Perhydroquinoxalines 3–5 reduced the release of Ca2+ ions into the cytoplasm after stimulation of HaCaT cells with ATP, thereby proving biological activity in human skin cells.

 Please wait while we load your content...
Please wait while we load your content...