Eliminating caspase-7 and cathepsin B cross-reactivity on fluorogenic caspase-3 substrates†
Abstract
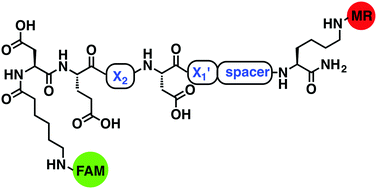
11 FRET-based fluorogenic substrates were constructed using the pentapeptide template Asp-Glu-X2-Asp-X1′, and evaluated with caspase-3, caspase-7 and cathepsin B. The sequence Asp-Glu-Pro-Asp-Ser was able to selectively quantify caspase-3 activity in vitro without notable caspase-7 and cathepsin B cross-reactivity, while exhibiting low μM KM values and good catalytic efficiencies (7.0–16.9 μM−1 min−1).
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...