The discovery of new acetylcholinesterase inhibitors derived from pharmacophore modeling, virtual screening, docking simulation and bioassays
Abstract
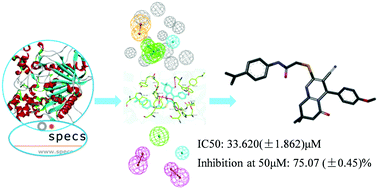
Hyperactivity of acetylcholinesterase (AChE) in the brain is the immediate cause of Alzheimer's disease (AD), which is one of the most prevalent fatal diseases afflicting numerous older people. In this research, an in silico study was carried out to find potential AChE inhibitors from a large chemical library. With clustering and lots of comprehensive analysis, some molecules were screened using in vitro bioassays. The IC50 values against AChE ranged from 33.620 to 101.570 μM, while the inhibition ratios at 50 μM ranged from 11.37% to 77.35%. The binding mode between the inhibitor and the binding sites of AChE was studied. Four residues (Tyr133, Tyr124, Ser203 and Trp86) were suggested to be crucial because they can form hydrogen bonds with the ligand. Therefore, ZYQ1 and its derivatives might represent a promising starting point for the development of highly potent lead compounds for the treatment of AD.

 Please wait while we load your content...
Please wait while we load your content...