Structural analysis of oncogenic mutation of isocitrate dehydrogenase 1
Abstract
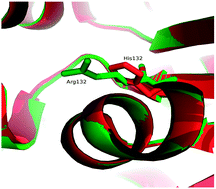
Arginine to histidine mutation at position 132 (R132H) in isocitrate dehydrogenase 1 (IDH1) led to reduced affinity of the respective enzymes for isocitrate and increased affinity for α-ketoglutarate (AKG) and NADPH. This phenomenon retarded oxidative decarboxylation of isocitrate to AKG and conferred a novel enzymatic activity that facilitated the reduction of AKG to D-2-hydroxyglutarate (D-2HG). The loss of isocitrate utilization and gain of 2HG production from IDH1 R132H had been taken up as a fundamental problem and to solve this, structural biology approaches were adopted. Interaction analysis was carried out to investigate the IDH1 substrate binding environment. The altered behaviour of mutant and native IDH1 in interaction analysis was explored by performing long-term molecular dynamics simulations (∼300 ns). This study reports a comprehensive atomic behaviour of the gain-of-function mutation (R132H) in the IDH1 enzyme which in turn provides a direction towards new therapeutics.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...