Mechanistic insights into the phosphatidylinositol binding properties of the pleckstrin homology domain of lamellipodin†
Abstract
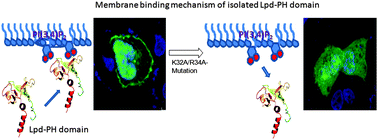
Lamellipodin (Lpd) protein plays an important role in the formation of lamellipodial protrusion which is crucial in actin dynamics, cell polarity and motility. Lpd promotes actin polymerization with the help of members of the Ena/VASP family of actin regulators and tethering them to actin filaments. It is well documented that Lpd protein interacts with the membrane containing phosphatidylinositols through its pleckstrin homology (PH) domain and regulates several cellular functions and cell migration. However, the molecular mechanism that underlies how the PH domain of Lpd specifically gets recruited to phosphatidylinositols remains unclear. To understand their interaction properties, we quantitatively determined the binding parameters of the Lpd-PH domain employing a number of biophysical studies including surface plasmon resonance (SPR), fluorescence resonance energy transfer (FRET)-based competitive binding assay and monolayer penetration measurements. Our studies showed that the Lpd-PH domain strongly interacts with PI(3,4)P2 containing liposome without any membrane penetration. Mutational studies demonstrate that the presence of cationic residues within the phosphatidylinositol (PIP) binding site of the Lpd-PH domain is essential in membrane binding. The translocation patterns of the Lpd-PH domain and mutants in platelet-derived growth factor (PDGF) stimulated A549 cells are in good agreement with our in vitro binding measurements. Overall, these studies demonstrate an insight into how the Lpd-PH domain regulates cellular signals in a PI(3,4)P2 dependent manner.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...