Highly efficient adenoviral transduction of pancreatic islets using a microfluidic device†
Abstract
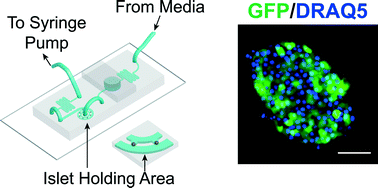
Tissues are challenging to genetically manipulate due to limited penetration of viral particles resulting in low transduction efficiency. We are particularly interested in expressing genetically-encoded sensors in ex vivo pancreatic islets to measure glucose-stimulated metabolism, however poor viral penetration biases these measurements to only a subset of cells at the periphery. To increase mass transfer of viral particles, we designed a microfluidic device that holds islets in parallel hydrodynamic traps connected by an expanding by-pass channel. We modeled viral particle flow into the tissue using fluorescently-labelled gold nanoparticles of varying sizes and showed a penetration threshold of only ∼5 nm. To increase this threshold, we used EDTA to transiently reduce cell–cell adhesion and expand intercellular space. Ultimately, a combination of media flow and ETDA treatment significantly increased adenoviral transduction to the core of the islet. As proof-of-principle, we used this protocol to transduce an ER-targeted redox sensitive sensor (eroGFP), and revealed significantly greater ER redox capacity at core islet cells. Overall, these data demonstrate a robust method to enhance transduction efficiency of islets, and potentially other tissues, by using a combination of microfluidic flow and transient tissue expansion.

 Please wait while we load your content...
Please wait while we load your content...