Mass bias stabilization by Mg doping for Si stable isotope analysis by MC-ICP-MS
Abstract
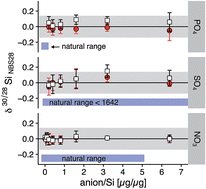
Controlling the mass bias during multi-collector ICP mass spectrometric measurements is fundamental to obtain accurate and precise metal and metalloid stable isotope ratios. When measuring Si stable isotopes under ‘dry’ plasma conditions, the instrumental mass bias is highly sensitive to remaining anionic and cationic impurities after chromatographic Si purification. Such impurities induce a matrix load that differs between samples and the standards used to correct for mass bias and thus impair the precision and accuracy of isotope ratio results obtainable by the standard sample bracketing technique. The addition of Mg under ‘dry’ plasma conditions reduces variations in the instrumental mass bias. Tests reveal that Mg addition (1) increases the Si ion intensity compared to Mg-free solutions, (2) allows a higher tolerance in the mismatching of Si and Mg concentrations between samples and bracketing standards to ±50% with no effect observed on instrumental mass bias, (3) allows us to vary the HCl molarity in samples from 0.05 to 0.2 mol l−1 HCl measured against a 0.1 mol l−1 HCl bracketing standard solution with no observable changes in the instrumental mass bias and (4) ensures that remaining anionic impurities (PO43−, SO42−, and NO3−) have no effect on the mass bias if the mass ratio of [anions] to Si is lower than 1. Therefore, the addition of Mg is the method of choice for Si isotope determination under ‘dry’ plasma conditions.


 Please wait while we load your content...
Please wait while we load your content...