Usability of online-coupling ion exchange chromatography ICP-AES/-MS for the determination of trivalent metal complex species under acidic conditions
Abstract
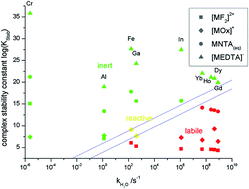
The determination of metal complex species in aqueous solutions by using chromatographic techniques is potentially affected or even impossible due to species decomposition during the separation. An often used technique for slower exchanging metal ions is ion exchange chromatography (IC), which is used for the separation and quantification of 1-1-complexes of trivalent metal ions. The test set consisted of chelating agents F−, Ox2−, NTA3− and EDTA4−, differing in their denticity, and the trivalent metals ions of Cr, Al, Fe, Ga, In and lanthanoids differing in their ligand exchange rate. It became apparent that the ligand exchange rate of the metal ion and the denticity of the chelator both play an important role. For slow exchanging metals and/or high denticity ligands, IC is a suitable tool for the determination of species distributions. A simple empirical equation is given to distinguish between inert, e.g. suitable for chromatographic separations, and labile 1-1-complexes of trivalent metal ions by using their complex formation constants and the aqua ligand exchange rate of the metal ions.

 Please wait while we load your content...
Please wait while we load your content...