Electrochemically initiated formation of sulfonyl radicals: synthesis of oxindoles via difunctionalization of acrylamides mediated by bromide ion†
Abstract
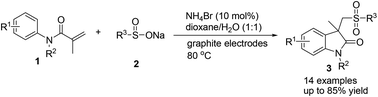
An efficient electrochemical approach for the synthesis of oxindoles has been developed from electrolysis of a mixture of sodium sulfinate and acrylamide. The chemistry avoids the utilization of additional conducting salt and proceeds in a simple undivided cell employing a catalytic amount of NH4Br (10 mmol%) as a redox catalyst. The protocol is practical, as proved by the one-pot, two-step procedure and scaled-up experiments. Mechanistic studies reveal that the electrochemical approach involves the formation of sulfonyl radicals via homolytic cleavage of in situ generated sulfonyl bromide.

 Please wait while we load your content...
Please wait while we load your content...