Non-isocyanate poly(amide-hydroxyurethane)s from sustainable resources†
Abstract
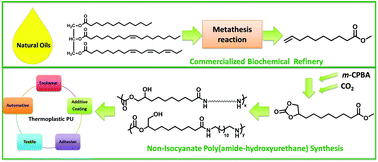
A two-step synthesis of epoxidation and carbonation afforded a hetero-functional AB monomer with cyclic carbonate and methyl ester (CC-ME) using plant oil-based methyl 9-decenoate and CO2. A unprecedented one-pot synthetic platform of CC-ME with 1,12-diaminododecane and poly(tetramethylene oxide) (PTMO)-based polyether diamine allowed synthesis of both nonsegmented poly(amide-hydroxyurethane) (PA12HU) and segmented PA12HU-PTMOs with varying polyether contents. 1H NMR spectroscopy confirmed complete conversion of cyclic carbonates and methyl esters to hydroxyurethanes and amides, respectively. Thermal analysis revealed distinctive thermal stability and transitions of PA12HU and PA12HU-PTMOs compared to their precursors and model oligomers. PA12HU and PA12HU-PTMOs were melt compression molded into semicrystalline, free-standing films, except for PA12HU-PTMO100 with 100% polyether diamine. PA12HU-PTMO100 was a viscous liquid with a glass transition temperature (Tg) of −64 °C and zero-shear melt viscosity of 449 Pa s. PA12HU formed a semicrystalline, rigid film with Tg of 11 °C. Polyether incorporation afforded creasable PA12HU-PTMO films with broad glass transitions near −50 °C. Thermal and thermomechanical analysis revealed significant phase-mixing of the hard and soft segments from annealed PA12HU-PTMO films. Polyether soft segments mixed with the amorphous hard segments, forming a miscible soft phase; crystallizable hard segments with ordered hydrogen bonding formed a hard phase. Surface morphological analysis of each PA12HU-PTMO film displayed ribbon-like, hard domains with composition-dependent aspect ratios. PA12HU-PTMOs exhibited higher moisture uptake than traditional thermoplastic polyurethane (TPU) due to resultant hydroxyls. Variable temperature FTIR spectroscopy demonstrated that ordered hydrogen bonding in the crystalline domains was disrupted or dissociated as the crystallites melted. Although tensile strength of segmented PA12HU-PTMOs proved lower than traditional polyurethanes due to phase-mixing, these compositions represent the first examples of film-forming, linear isocyanate-free polyurethanes with mechanical integrity and processability.

 Please wait while we load your content...
Please wait while we load your content...