Understanding the cleavage of inter- and intramolecular linkages in corncob residue for utilization of lignin to produce monophenols†
Abstract
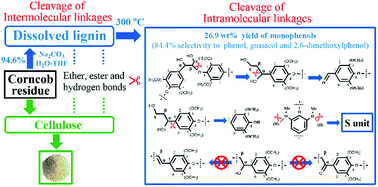
The cleavage of inter- and intramolecular linkages is a crucial but complicated issue for isolation and depolymerization of lignin in lignocellulosic biomass to produce biofuel. Herein, a Na2CO3–H2O–tetrahydrofuran system was adopted for studying the cleavage of these linkages in corncob residue. Almost all ether, ester and hydrogen bonds between lignin and cellulose were broken by Na2CO3 at 140 °C, resulting in 94.6% dissolution of lignin, meanwhile the C–O bond in the β-O-4 linkage and the Cα–Cβ bond in the aliphatic side-chain of lignin could be broken to obtain aryl aldehydes. At 300 °C, the CAr–Cα bond could be broken down, bringing about high selectivity to monophenols without substituted alkyl groups, and the total yield of monophenols reached up to 26.9 wt% without additional hydrogen. Na2CO3 could also inhibit the formation of a Cα-oxidized side-chain product and the elimination reaction of an –OH group on Cα forming p-coumaric acid. G and H units in the corncob residue were mostly connected by a β-O-4 linkage, whereas S units were possibly linked by an α-O-4 linkage. The hydrogen bonds at –OCH3 groups in S and G units in lignin could be disrupted by Na2CO3 to facilitate the dissolution and degradation of lignin.

 Please wait while we load your content...
Please wait while we load your content...