Solvent-free synthesis of quaternary α-hydroxy α-trifluoromethyl diazenes: the key step of a nucleophilic formylation strategy†
Abstract
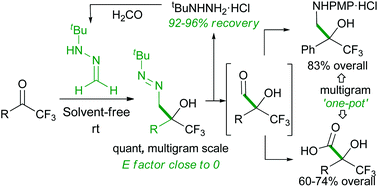
An efficient, scalable and operationally simple one-pot, 2-step strategy for the nucleophilic formylation of trifluoromethyl ketones is presented. The key step is an unprecedented diaza-carbonyl-ene reaction of formaldehyde tert-butyl hydrazone and trifluoromethyl ketones under solvent-free conditions. This reaction proved to be very fast, clean and high-yielding, affording densely functionalised α-hydroxy α-trifluoromethyl diazenes. The ensuing diazene-to-aldehyde transformation, avoiding protection/deprotection reactions and chromatographic purifications, and subsequent derivatizations in a one-pot fashion provide a direct entry to a variety of useful trifluoromethylated building blocks.

 Please wait while we load your content...
Please wait while we load your content...