Industrially scalable and cost-effective synthesis of 1,3-cyclopentanediol with furfuryl alcohol from lignocellulose†
Abstract
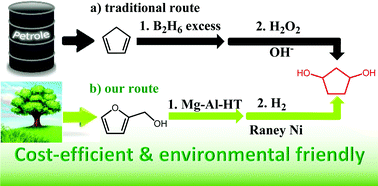
A new route for the selective synthesis of renewable 1,3-cyclopentanediol was developed by the aqueous phase rearrangement of furfuryl alcohol to 4-hydroxycyclopent-2-enone followed by hydrogenation. The presence of a small amount of base catalysts is beneficial for the aqueous phase rearrangement of furfuryl alcohol to 4-hydroxycyclopent-2-enone. Such a promotion effect of base catalysts can be rationalized by restraining the generation of levulinic acid which may catalyze the polymerization of furfuryl alcohol. In the hydrogenation of 4-hydroxycyclopent-2-enone to 1,3-cyclopentanediol, an evident solvent effect was noticed. Higher carbon yields of 1,3-cyclopentanediol were obtained when tetrahydrofuran was used as the solvent. In the large scale tests with high initial concentrations of feedstocks, a high overall carbon yield (72.0%) of 1,3-cyclopentanediol was achieved over cheap catalysts (MgAl-HT and RANEY® Ni). As a potential application, 1,3-cyclopentanediol as obtained was successfully used as a monomer in the synthesis of polyurethane.

 Please wait while we load your content...
Please wait while we load your content...